N-Heterocyclic carbene catalysis
The use of catalysts to promote reactions involving umpolung intermediates is a key research interest. Recently we have focused (along with Dr. Kirsten Zeitler in Regensburg) on attempting to solve the 175+ year old problem of how to stereo- and chemoselectively couple two different carbonyl compounds in a acyloin condensation process.
'Organocatalytic aerobic oxidative cleavage of cyclic 1,2-diketones'
S. Gundala, C. -L. Fagan, E. G. Delany and S. J. Connon*, Synlett 2013, 1225.
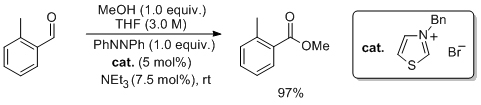
'Aerobic oxidation of NHC-catalysed aldehyde esterifications with alcohols: benzoin, not the Breslow intermediate, undergoes oxidation'
E. G. Delany, C. -L. Fagan, S. Gundala, K. Zeitler* and S. J. Connon*, Chem. Commun. 2013, 49, 6513.
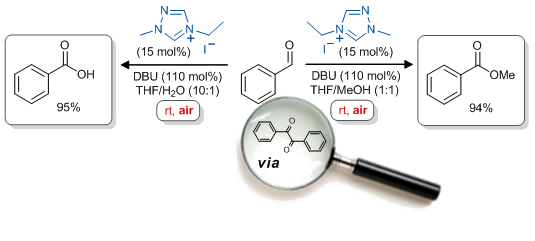
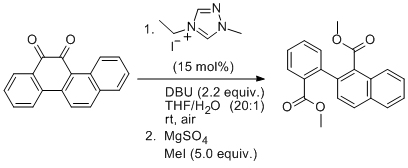
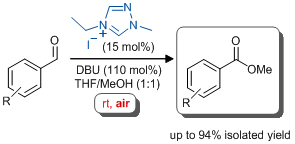
'NHC-catalysed aerobic aldehyde-esterifications with alcohols: no additives or cocatalysts required'
E. G. Delany, C. -L. Fagan, S. Gundala, A. Mari, T. Broja, K. Zeitler* and S. J. Connon*, Chem. Commun. 2013, 49, 6510.
'NHC-Catalysed, chemoselective crossed-acyloin reactions'
C. A. Rose, S. Gundala, C. -L. Fagan, J. F. Franz, S. J. Connon* and K. Zeitler*, Chem. Sci. 2012, 3, 735.
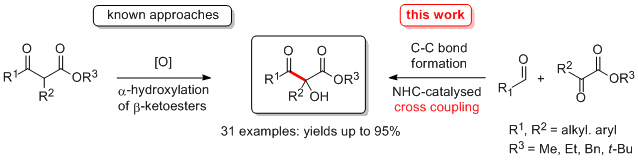
'Chemoselective crossed acyloin condensations: catalyst and substrate control'
C. A. Rose, S. Gundala, K. Zeitler* and S. J. Connon*, Synthesis 2011, 190.
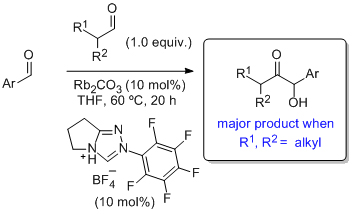
'Highly chemoselective direct crossed aliphatic-aromatic acyloin condensations with triazolium-derived carbene catalysts'
S. E. O'Toole, C. A. Rose, S. Gundala, K. Zeitler* and S. J. Connon*, J. Org. Chem. 2011, 76, 347.
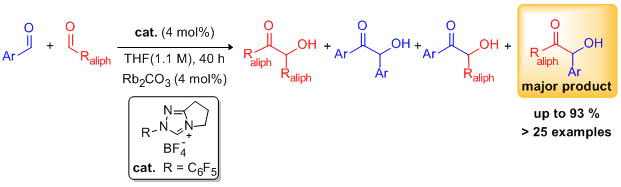
'Highly enantioselective benzoin condensation reactions involving a bifunctional protic pentafluorophenyl-substituted triazolium precatalyst'
L. Baragwanath, C. A. Rose, K. Zeitler and S. J. Connon*, J. Org. Chem. 2009, 74, 9214.
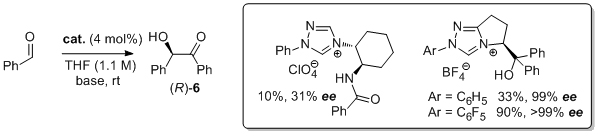
'The enantioselective benzoin condensation promoted by chiral triazolium precatalysts: stereochemical control via hydrogen bonding'
S. E. O'Toole and S. J. Connon*, Org. Biomol. Chem. 2009, 7, 3584.
'Nucleophilic carbene-catalysed oxidative esterification reactions'
C. Noonan, L. Baragwanath and S. J. Connon*, Tetrahedron Lett. 2008, 49, 4003.